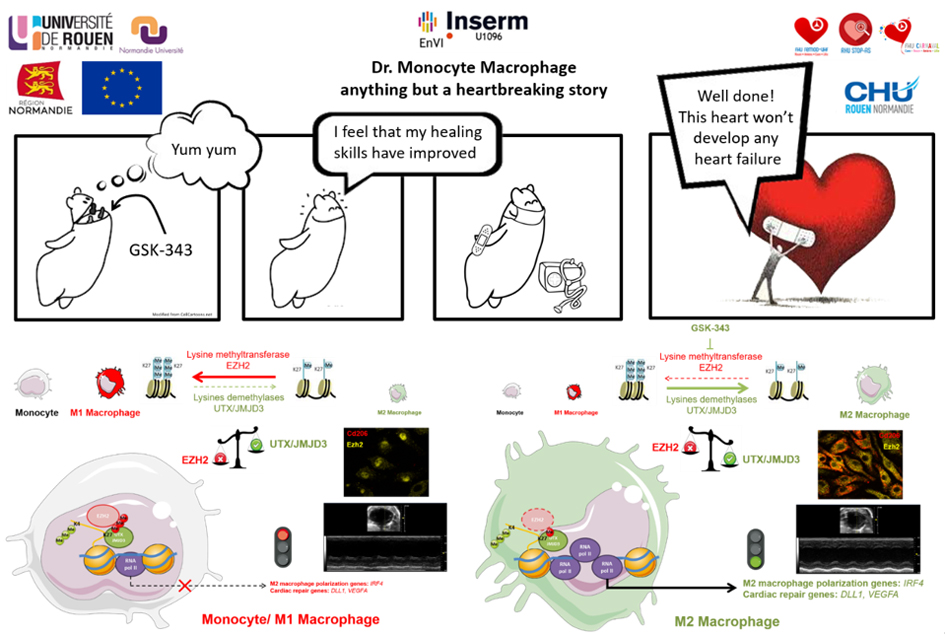
Epigenetic check-point regulates polarization of monocytes post-MI
Myocardial infarction (MI) triggers a wound healing response that involves rapid recruitment of immune cells, notably myeloid cells, to the heart. This innate immune response is required for efficient healing, and alteration of its kinetics may contribute to poor infarct remodeling driving heart failure development. In the latest issue of Nature Communications, the team led by Dr. Fraineau described for the first time the role of an epigenetic switch in regulating the kinetics and profiles of the innate immune response post-MI. Specifically, they uncovered that an epigenetic transcriptional repressive enzyme, named EZH2, serves as an epigenetic check-point during polarization of monocytes toward an immunomodulatory pro-regenerative macrophage phenotype. Using an epigenetic pharmacological inhibitor of EZH2, this check-point can be suppressed, thus enhancing the transcriptomic program resulting in generation of more immunomodulatory macrophages. This led to an acceleration of the innate immune response after MI, resulting in reduced deleterious infarct scar remodeling, and prevention of post-MI aggravation of cardiac dysfunction. Altogether, Fraineau et al. identified an original epigenetic mechanism governing macrophage polarization toward either proinflammatory or immunomodulatory phenotypes, thus revealing epigenetic mechanisms as a novel therapeutic target to improve inflammatory kinetics post-MI to prevent heart failure development.
See further: open access article or video presentation by Dr Fraineau