Latest News
Junior Research Chair - Translational research in Valvulopathy
Position announcement
Valvular heart diseases are common and increasing in prevalence due to population aging, with an incidence comparable to that of Alzheimer’s disease. Their pathophysiology remains poorly understood, and no treatment currently exists to halt or slow their progression. No structure in France or Europe currently has the capacity to develop translational and multidisciplinary research programs on these diseases with the scope and ambition envisioned by the Valve Institute. Building on ten years of collaborative experience (FHU/RHU), the Alain Cribier Institute aims to bring together all the key players in the field of cardiac valves on a single site by:
- Accelerating translational research to benefit patients,
- Generating world-class scientific innovations and publications,
- Attracting young scientists, physicians, and pharmacists.
We are now seeking a talented early-career researcher (4-10 years experience, including PhD) for a Junior Chair Position (CPJ) for a duration of 4-6 years in our laboratory for the project "Development of innovative treatments for the management of cardiac valvular diseases". In addition to their support for salary (set according to national university rules), the holder of the CPJ will receive a research grant of €200,000, allowing for the recruitment of a technician for 3 years (€105,000) and for covering operating and travel expenses up to €85,000 and €10,000 respectively.
Prerequisite for applicants:
The CPJ candidate should have postdoctoral experience. They will be responsible for coordinating, leading, organizing, and energizing preclinical and translational research in the field of therapeutic innovation for the management of valvular heart diseases—particularly calcified aortic stenosis—aligned with Axis 2 of INSERM Unit 1096, in collaboration with the Cardiology and Cardiothoracic Surgery Departments of the Rouen University Hospital. Specifically, the CPJ candidate should have expertise in both cell culture and animal experimentation in relation to translational cardiovascular research. Their responsibilities will include:
- Managing the isolation and study of valvular cells
- Organotypic culture of murine, porcine, or human explanted valves
- In vivo studies in murine models of aortic stenosis, particularly in contexts of kidney failure induced by nephrectomy or metabolic disorders
- Establishing methodologies for studying bioprosthetic valve degeneration in rodents (subcutaneous implantation)
- Setting up a large animal model (sheep or pig) for in vivo investigation of the mechanisms and surgical or pharmacological treatments of aortic stenosis
- Leading and conducting biological studies in support of clinical trials and cohort studies carried out within the Institute.
In addition, the CPJ should participate and lead activities related to teaching (48h annually, in French). The pedagogical objective for the CPJ will be to develop cardiovascular education within the Faculty of Health (UFR Santé), as well as more broadly across the University of Rouen Normandy, with a particular focus on valvular heart diseases, both from theoretical and practical perspectives, including:
- Strengthening teaching teams across the departments of the Faculty of Health (Medicine, Pharmacy, Speech Therapy, Dentistry, Rehabilitation Sciences, etc.) for both mandatory and elective courses focused on the pathophysiology and therapeutic approaches to cardiovascular diseases
- Establishing a new University Diploma (DU/DIU) dedicated to valvular diseases
- Developing new teaching approaches based on simulation, in direct collaboration with the
Medical Training Center (MTC) - Participating in teaching activities related to cell biology, molecular biology, and pathophysiology within the various academic units of the University of Rouen Normandy (Faculty of Science and Technology, ESITECH, IUT, etc.)
How to apply:
Candidates must register their application and mandatory attach the documents constituting their file in .pdf format on the website of the Ministry of Higher Education, Research and Innovation (MESRI), via the ODYSSEE application. Me connecter - ODYSSÉE Position ID: 252923
https://euraxess.ec.europa.eu/jobs/345569
Candidates must prepare a file composed of:
- an application form completed online
- a digital version of the following documents:
- a photo ID;
- a document attesting to the possession of a doctorate, as provided for in Article L.612-7 of the Education Code, or a diploma whose equivalence will be recognised in accordance with the procedure set out in 1° of Article 5 of the above-mentioned Decree of 17 December 2021; « report on the defence of the diploma produced;
- analytical presentation of the work, books, articles and achievements made on the model of the "CPJ application form" to be submitted as document 1 in the "titles and works";
- main titles and works indicated in the analytical presentation.
The administrative documents and the defence report written in whole or in part in a foreign language are accompanied by a translation into French, the conformity of which the candidate certifies on their honour. Otherwise, the file is declared unacceptable. The translation of the analytical presentation as well as of the works, books, articles and achievements is optional. All these documents must be submitted in digital version by the date indicated in the recruitment notice at the latest. Any application that is incomplete by the above-mentioned deadline is declared unacceptable. Only persons previously selected on the basis of their application by the selection committee, whose composition will be made public before the start of its work, will be invited to the audition. All applicants will access the status of their application and the results using the Odyssey candidate number and personal password. Any candidate selected for one or more positions at the end of the procedure will have to commit on the dedicated application to hold the position.
Contact : Jérémy Bellien,
Open science practices
The dissemination strategy of the DIVE project is designed to reach a wide audience, including the scientific community, healthcare professionals, and the general public. Scientific dissemination will take place through presentations at international congresses, such as those of the European Society of Cardiology (ESC), as well as through publications in high-impact, peer-reviewed journals such as Circulation, European Heart Journal, and potentially in multidisciplinary journals such as Nature Communications, ensuring open-access availability.
Science-to-practice communication will rely on direct engagement with healthcare professionals and students through university-level teaching and partnerships with clinical institutions, thereby facilitating the transfer of knowledge to physicians and patients. Locally, dissemination will involve participation in scientific events organized in Rouen, such as the annual Scientific Day dedicated to clinical and basic cardiovascular research, held within the framework of the CARNAVAL program, which received the Fédération Hospitalo-Universitaire (FHU) label in 2020. At the national level, the project will be represented at the annual meetings of the French Society of Cardiology and the French Society of Pharmacology and Therapeutics, fostering the development of multidisciplinary scientific networks and sharing project outcomes across both communities.
Firmly committed to open science, the project carried by the CPJ will adhere to the principles of transparency, open access, and knowledge sharing. All scientific publications resulting from the project will be made available in open access, in line with recommendations from INSERM and European funding agencies, to ensure immediate and unrestricted access to results for the scientific community, healthcare professionals, and the general public.
- All articles will be systematically deposited in the national open archive HAL, in addition to being published in peer-reviewed international journals. Data generated by the project will be stored in certified repositories such as Zenodo or the European Nucleotide Archive, accompanied by FAIR (Findable, Accessible, Interoperable, Reusable) metadata to ensure reusability by other researchers.
- Source code and experimental protocols will also be shared via open platforms such as GitHub and protocols.io.
- Public outreach activities will include media campaigns and social media engagement, as well as interactive events such as workshops and public lectures to raise awareness of the project's clinical relevance. Partnerships with patient associations, along with participation in community events such as Patient Information Days and Pint of Science, will help ensure broad dissemination of knowledge. Media appearances -— including press articles, commentaries in medical forums, and television interviews — will be supported by INSERM’s Press Office, which produces press releases and scientific highlights. A dedicated website for the project will also be created and hosted on the laboratory's homepage (http://www.insermu1096.fr/), ensuring ongoing and accessible communication with both the scientific community and the general public
Mohammad Al Tarrass
 |
|
| PhD in Cell Biology | |
| LinkedIn: Mohammad-al-tarrass ; ORCID: 0009-0003-9337-9100 |
Biography
Mohammad Al Tarrass is a passionate early-stage researcher with a strong background in biochemistry, cell biology, and expertise in vascular biology. He holds a Bachelor's degree in Biochemistry from Lebanese University and completed a double diploma Master’s degree in Healthy Living Technologies with honors in 2019, through a collaborative program between Lebanese University (Lebanon) and the University of Grenoble Alpes (France). During this program, he performed his Master's internship at the Biomics Lab (CEA Grenoble), where he investigated the DNA-dependent protease SPRTN as a therapeutic target in cancer.
Following this, Mohammad began his PhD at CEA Grenoble-Biosanté, working within the BMP family in the angiogenesis and lymphangiogenesis team (BAL). His research focused on deciphering phosphoproteomic changes in response to BMP9 and BMP10 in endothelial cells. The aim was to understand the role of these ligands in vascular homeostasis and propose novel therapeutic approaches for treating rare vascular diseases, particularly Hereditary Hemorrhagic Telangiectasia (HHT) and Pulmonary Arterial Hypertension (PAH), which are associated with defects in their signaling pathways. He successfully obtained his PhD in Cell Biology from the University of Grenoble Alpes in 2023.
Throughout his scientific career, Mohammad has gained significant experience in various aspects of cellular biology, utilizing a wide range of molecular and biochemical techniques alongside advanced technologies (Mass-spectrometry). He has published three scientific papers, a book chapter, and presented his research at several national and international conferences (Researchgate).
In addition to his role in the MIMOSA project, Dr Al Tarrass is developing and validating, together with Dr Thomas Duflot, an analytical method for analyzing sphingosine and sphingosine-1-phosphate (S1P) species in biological samples (in vivo or in vitro models of cardiovascular disease) using liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). He will also investigate the role of S1P signaling in vascular endothelial cells and its significance in maintaining vascular and lymphatic barriers. This work builds on ongoing international projects led by the EnVI laboratory, including ANR PHASM (Dr Jeremy Bellien) and ANR CITE-LYMPH (Dr Ebba Brakenhielm).
Inserm U1096 in the news
Previous media articles on our research:
- Memory services for Pr Alain Cribier Décès du Professeur Alain Cribier - Université de Rouen Normandie (univ-rouen.fr), Hommage au Professeur Alain Cribier (chu-rouen.fr)
- Highlight on chronic kidney disease by lab member Pr D Guerrot
- Highlight of our preclinical research in The Conversation
- Projet FHU CARNAVAL
- FASEB BIOART 2020
- RHU STOP-AS (@RHU_STOPAS) | FDNitter
- Projet RHU STOP-AS sélectioné
- Un reseau invisible au secours du coeur
- Prix Jean-Paul Binet
- Ma thèse en 180 secondes
- Prix "Don de soi -don de vie"
- Réseau ERA-CVD LYMIT-DIS
- Réseau FHU REMOD-VHF
- Helene Eltchaninoff, membre de la Fédération Hospitalo-Universitaire - Portail vidéo de l'Université de Rouen Normandie (univ-rouen.fr)
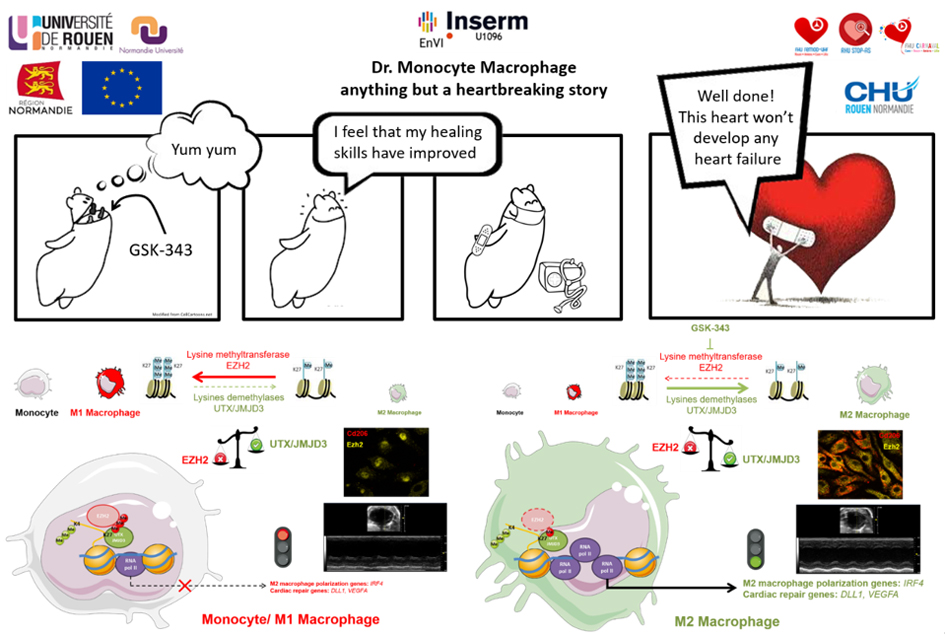
Epigenetic check-point regulates polarization of monocytes post-MI
Myocardial infarction (MI) triggers a wound healing response that involves rapid recruitment of immune cells, notably myeloid cells, to the heart. This innate immune response is required for efficient healing, and alteration of its kinetics may contribute to poor infarct remodeling driving heart failure development. In the latest issue of Nature Communications, the team led by Dr. Fraineau described for the first time the role of an epigenetic switch in regulating the kinetics and profiles of the innate immune response post-MI. Specifically, they uncovered that an epigenetic transcriptional repressive enzyme, named EZH2, serves as an epigenetic check-point during polarization of monocytes toward an immunomodulatory pro-regenerative macrophage phenotype. Using an epigenetic pharmacological inhibitor of EZH2, this check-point can be suppressed, thus enhancing the transcriptomic program resulting in generation of more immunomodulatory macrophages. This led to an acceleration of the innate immune response after MI, resulting in reduced deleterious infarct scar remodeling, and prevention of post-MI aggravation of cardiac dysfunction. Altogether, Fraineau et al. identified an original epigenetic mechanism governing macrophage polarization toward either proinflammatory or immunomodulatory phenotypes, thus revealing epigenetic mechanisms as a novel therapeutic target to improve inflammatory kinetics post-MI to prevent heart failure development.
See further: open access article or video presentation by Dr Fraineau